![]() Aerosol based Production and Passivation Submicron Aluminium Particles
Aerosol based Production and Passivation Submicron Aluminium Particles
PI: Prof. Michael R Zachariah
We show a low temperature gas-phase synthesis route to produce faceted aluminum crystals in the aerosol phase. Use of triisobutylaluminum whose decomposition temperature is below the melting point of elemental aluminum enabled us to grow nanocrystals from its vapor. TEM shows both polyhedral crystalline and spherical particle morphologies, but with the addition of an annealing furnace one can significantly enhance production of just the polyhedral particles. The results on surface passivation with oxygen suggest that these nanocrystals are less pyrophoric than the corresponding spherical aluminum nanoparticles. The figure below shows the formation of such particles.
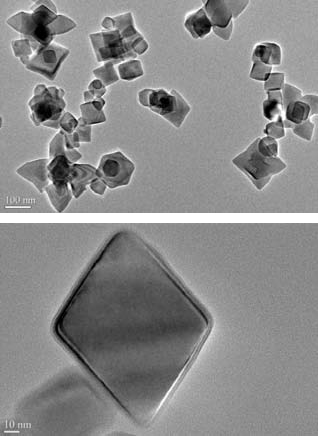
TEM images of Al Nanocrystals
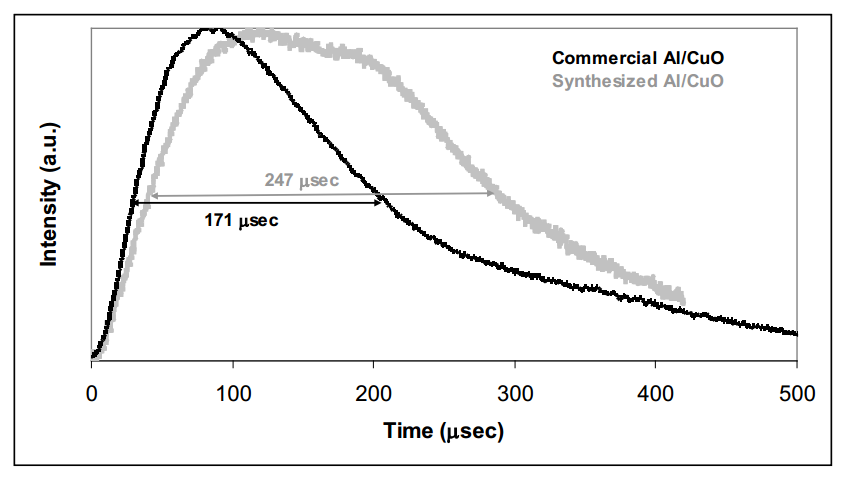
To test for reactivity of the polyhedral crystalline particles, samples were combined with stoichiometric CuO and burned in a pressure cell. The resulting optical response is compared to a response from commercial aluminum/CuO in the figure below. The broader peak for the synthesized aluminum suggests that either the polyhedral crystalline particles ignite at a higher temperature after the spherical particles in the sample have started
burning, or the polyhedral particles actually have longer burn times than standard nanoaluminum. This result is consistent with the previous observation that the polyhedral crystal particles were not as pyrophoric as spherical particles produced with our system. The enhanced stability of these particles can be attributed to the higher surface binding energy for molecules on a flat surface compared to that of a curved surface due to the Kelvin
effect.
Pressure response from these experiments showed a maximum pressure rise of 166 psi for the synthesized Al compared to a value of 116 psi for commercial product, both with similar rise times. The polyhedral particles thus yield a significant enhancement in reactivity. This information combined with the optical and experimental observations lead to the conclusions that we have produced polyhedral nanoaluminum particles with both enhanced stability and increased energy release.
A paper on this work:
Dan A. Kaplowitz, R.J. Jouet, Michael R. Zachariah, "Aerosol synthesis and reactive behavior of faceted aluminum nanocrystals", Journal of Crystal Growth, Volume 312, Issue 24, 1 December 2010, Pages 3625-3630.

