![]() Ultra Sensitive Detection of Low-Vapor Pressure Compounds by Ion-Nucleation Detection (INDe)
Ultra Sensitive Detection of Low-Vapor Pressure Compounds by Ion-Nucleation Detection (INDe)
PI: Prof. Michael Zachariah
We are working to develop an ultra-sensitive method (sub-PPT) for nearly single-molecule detection of explosives and other low-vapor pressure compounds, that will improve sensitivity by several orders of magnitude over traditional ion-counting. The basic concept relies on a new approach to measure the presence of a molecule. Unlike traditional mass-spectrometry or optical methods that rely on a direct transduction of an electrical signal coming from the analyte (which greatly limits sensitivity), our method relies on the very rapid gas-to-liquid transition an ion can induce in a super saturated environment to create a micron sized droplet with an ion as its seed. Our approach has the potential to measure molecules one at a time by using each molecule as the site for growth of a droplet. The resulting droplet, which contains one analyte species, can then be detected with a commercial particle counting apparatus or any other light scattering approach remotely located. We call this approach Ion Nucleation Detection (INDe). By incorporating this approach into traditional ion-mobility spectrometry (IMS), we expect to improve the sensitivity of the IMS by replacing the widely used Faraday plate sensor with a particle size magnifier (PSM) and a condensation particle counter (CPC).
A system including an atmospheric pressure ion-mobility spectrometer coupled to an electrospray source was developed to generate a steady source of ions of defined size. A first and ultimately second generation ion-magnifier was designed and constructed. The performance of a particle size magnifier (PSM) was tested using electro-sprayed tetra-alkyl ammonium ions and clusters thereof as nanoparticle standards, with diameters in the sub 2 nm range. Subsequent ion-mobility separation generates a continuous source of size selected ions.
The ions were detected by both a Faraday cup electrometer, and the PSM. Inside the PSM, the ion flow was turbulently mixed with a jet of a hot nitrogen flow, saturated with dibutyl phthalate (DBP). This allowed for heterogeneous nucleation of DBP onto the nanoparticles, which grew into droplets.
The performance of the PSM for tetraheptyl ammonium bromide (THAB) is shown in the figure below. The red curve shows the number concentrations of THAB as measured by the electrometer and the blue curve shows the PSM as a function of mobility diameter.
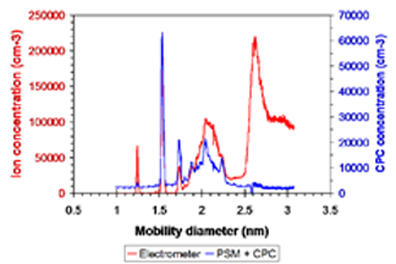
The INDe system is able to track the ion counts with the same precision as the electrometer implying that the concept has been demonstrated. With our current configuration, we find we lose sensitivity to detection to ~1.24 nm, while our target molecule, TNT is ~ 0.75 nm. To decrease the lower size limit and increase concentration sensitivity will require a redesign of the PSM. Currently, a new PSM is being tested that should improve the turbulent mixing rate and thus the separation between homogeneous and heterogene¬ous nucleation zones, such that we can operate at higher supersaturation ratios. We plan to investigate other working fluids besides DBP, which is currently used.

